About the study
generation hd 1 is a randomized, multicenter, double-blind, phase III, placebo-controlled clinical trial to evaluate the efficacy and safety of the drug RO7234292 (RG6042), administered intrathecally in symptomatic patients with Huntington's disease.
About the experimental drug
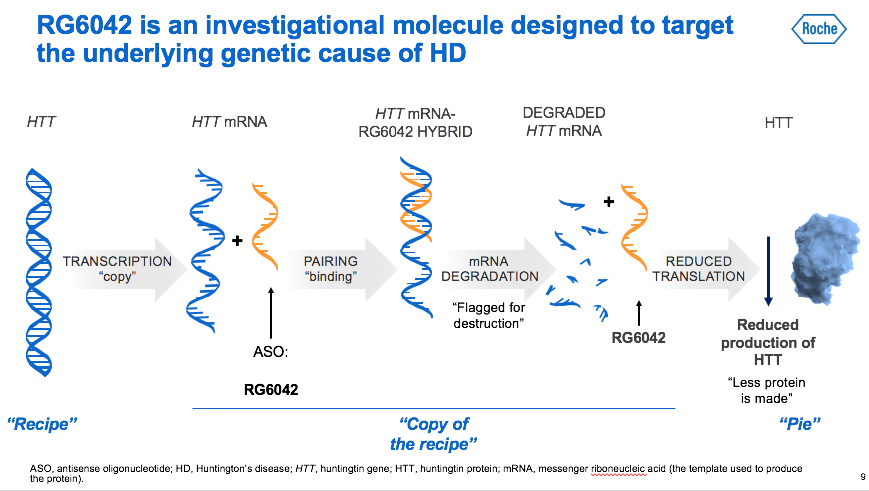
For the first time in the history of Huntington's disease, a molecule that directly affects the cause of the disease is tested on humans, reducing the messenger RNA that from the gene, promotes the formation of the huntingtin protein (mHTT). The HTT protein which, when mutated (mHTT), causes the neurons' death in some areas of the brain and, consequently, the onset and progression of the disease’s symptoms. The strategy used in the Generation HD 1 study is not selective: this means that both huntingtins are reduced: the mutated one, inherited from the carrier or affected parent, and the non-mutated one, inherited from the non-carrier or not sick parent.
about ASOs
Information about how our cells work are contained in DNA. DNA must be transcribed into messenger RNA so that this information can then be translated by the ribosomes used to "make" the proteins. The antisense drug is a small piece of synthetic DNA that pairs with messenger RNA and blocks its translation. In people with the mutant gene for huntingtin, ASOs are therefore aimed to block the production of messenger RNA which orders cells to produce the HTT and mHTT protein.
study sites
This is the largest clinical study conducted so far for Huntington's disease. It involves 801 participants, 19 Countries and over 100 sites worldwide.
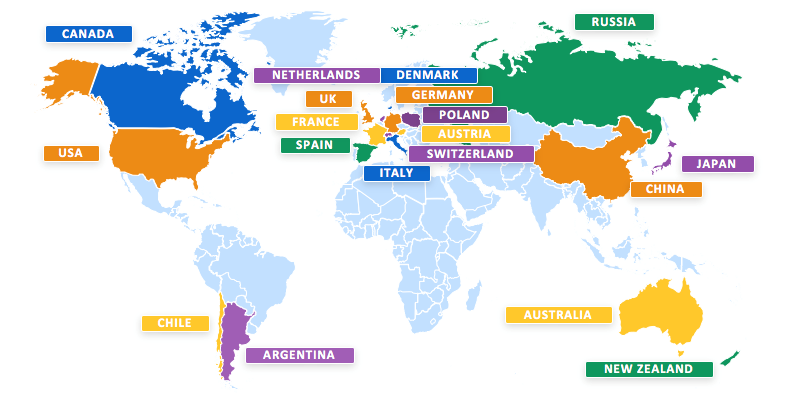
In Italy the study involves about 50 patients and is conducted in six centers:
- IRCCS Casa Sollievo della Sofferenza (CSS) - Principal Investigator: Ferdinando Squitieri
- IRCCS Istituto Neurologico Carlo Besta di Milano - Principal investigator: Caterina Mariotti
- Ospedale Sant’Andrea di Roma – Dipartimento di Neuroscienze - Principal Investigator: Giovanni Ristori
- Università degli Studi DINOGMI di Genova - Principal Investigator: Giovanni Abbruzzese
- Ospedale ISNB di Bologna - Principal Investigator: Pietro Cortelli
- Ospedale Careggi di Firenze - Principal Investigator: Alessandro Sorbi
Duration
The expected duration is 25 months plus follow-up. In Italy, phase III began in July 2019.
INCLUSION CRITERIA
Symptomatic patients in early stage of the disease can take part to the study. The specific criteria are:
- Genetic diagnosis of Huntington's disease
- Age 25-65
- CAP score> 400 (obtained from an equation that includes the age and length of the mutated section)
- Independence Scale ≥70
- Autonomy in walking, speaking , understanding and signing informed consent
drug administration
- 267 participants receive 120 mg of the drug every 2 months
- 267 participants receive 120 mg of drug every 4 months (every two months they receive placebo)
- 267 participants receive 120 mg of placebo every 2 months
- The study also provides for the use, not only during the visits but also daily at home, of smartphones and smartwatches.
drug administration
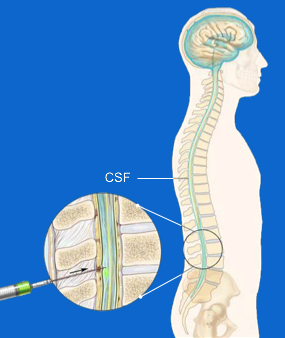
ASOs are unable to go directly to the brain due to the existence of a sort of 'defensive wall' that our body has developed around the central nervous system; this obstacle is called the 'blood brain barrier'. If on the one hand this system allows effective protection of our brain, on the other it requires that the drugs designed to act on the central neuro system are infiltrated in different way. RG6042 is an antisense therapy administered intrathecally, through a lumbar puncture in order to be injected directly into the liquid that surrounds the nervous system. Like this is able to act on it and overcome the "protective wall".
THE MOLECULE WILL BE AFFECTIVE IF...
... if it slows the progression of symptoms and improves patients’ autonomy.
The drug effectiveness indicators will be scored on the basis of some specific rating scales that measure the level of functional, cognitive and motor skills of those who took the drug, compared to those who took the placebo.
estimated conclusion of the study
mid 2022.
what else do we know about this drug?
Starting in August 2015, this drug was administered on a group of patients who took part to in the first phase of the study. 46 people were involved, to 34 of them was given the drug at increasing dose levels (from 10 mg to 120 mg); to the other 12 was given placebo. The study lasted six months, infusions of the investigative product were carried out every 28 days for the first four months, in the following months were monitored the trend of the concentration of the mutated protein in the CSF and the participants' health. During the study, no serious adverse events or clinically relevant adverse changes occurred in the laboratory analysis. The treatment with the drug led to a dose-dependent reduction in the concentration of the mHTT protein in the CSF (the average variations range from 20 to 42% at increasing dosage levels). This result confirms that the experimental product is able to reduce the concentration of mHTT.
So what do we not know yet?
Given the results of this study, we do not know whether the decrease in Huntingtin concentration recorded in the CSF is related to a decrease of its concentration in the central nervous system (although preclinical studies support this hypothesis). Furthermore, although there is a rational foundation for expecting positive clinical effects related to a decrease in the concentration of mHTT, there is still no evidence that associates it with a therapeutic effect on the disease's progression. This is why the third phase of the trial currently ongoing is so important:, it will tell us more about the effectiveness of the drug and it will allow us to continue monitoring its safety!

